 Hi I’m Dr. Sabrina Stierwalt and I’m Everyday Einstein bringing you Quick and Dirty Tips to help you make sense of science.
Hi I’m Dr. Sabrina Stierwalt and I’m Everyday Einstein bringing you Quick and Dirty Tips to help you make sense of science.
There are so many everyday uses for lasers, from dentistry and tattoo removal to printing and CD players (remember them?). They also pop up frequently in science fiction movies like in the famous light sabers of Star Wars.
However, lasers are no ordinary form of light. They are both monochromatic and coherent, which are special properties that allow us to use them in unique ways that would be impossible with a regular flashlight or a light bulb.
So what are lasers exactly? How do they work? Let’s look at how lasers can help us correct our vision and why I would rather fight someone with a light saber than a flashlight.
The Structure of an Atom
To understand how lasers work, we must first take a look at the atom. Everything we interact with on a daily basis – the chair you sit on, the air you breathe, even our bodies – are all made up of small particles called atoms. As shown in the periodic table of the elements, only about 100 different kinds of atoms exist. Different materials consist of different combinations of these elements.
See also: Atomic Bonds - The Ties That Bind
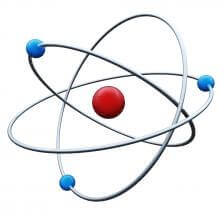 Each atom contains a nucleus (made up of protons and neutrons) and electrons that are constantly in motion to orbit the nucleus. Atoms have a ground level energy state, one that does not require any additional energy to maintain, where the electrons are orbiting closest to the nucleus. Those electrons can also be bumped or stimulated into higher energy level orbits so that the atom is considered to be in an “excited” energy state.
Each atom contains a nucleus (made up of protons and neutrons) and electrons that are constantly in motion to orbit the nucleus. Atoms have a ground level energy state, one that does not require any additional energy to maintain, where the electrons are orbiting closest to the nucleus. Those electrons can also be bumped or stimulated into higher energy level orbits so that the atom is considered to be in an “excited” energy state.
Thanks to quantum mechanics, we now know that this view of an atom is a bit simplified and that electrons aren’t likely to actually travel in discrete, well-defined orbits. However, such a picture is still a helpful description for relating the physics of small particles like electrons to the laws of physics we experience every day.
To excite an electron into a higher energy state, all that is needed is energy, usually in the form of light or heat.
When all those electrons decide to relax again, they can rejoin their neighbors in the lower energy levels when the atom releases energy in the form of photons or packets of light. The energy difference between the electron’s starting and ending orbits determines the energy of the photon that is released, which in turn sets the wavelength or color of the emitted light.
What Is a Laser and How Does it Work?
You may remember from Grammar Girl that the word “laser” is actually an acronym. It stands for Light Amplification by Stimulated Emission of Radiation. Doesn’t roll quite as easily off the tongue, does it?
The key word in all of that is “stimulated.” This is what sets lasers apart from more ordinary forms of light. When you turn on a normal flashlight, for example, light leaves in all directions and does so at random times. The resulting light is diffuse (or spread out) and relatively weak.
A laser, on the other hand, is “coherent” meaning that all of the photons leave in unison and in one direction. This kind of ordered exit results in a narrower, more intense beam of light.
So how do you get photons to follow such strict marching orders?
 Let’s go back to our picture of the atom. When an electron moves from an excited energy state back to the ground level, a photon with a very specific amount of energy is released. The energy of the photon that is produced is equal to the energy difference between the two energy levels (that is, between the ground and excited states of the atom).
Let’s go back to our picture of the atom. When an electron moves from an excited energy state back to the ground level, a photon with a very specific amount of energy is released. The energy of the photon that is produced is equal to the energy difference between the two energy levels (that is, between the ground and excited states of the atom).
If that newly released photon then encounters another electron sitting in the same excited energy state, it can trigger the second electron to also jump back down to the ground state by emitting a photon with the same energy (color) and phase (relative position).
If enough electrons are sitting in the excited state, the emission of that first photon can start off a chain reaction of stimulated emission. More photons are emitted when electrons move back down to their ground level energy states and those newly emitted photons in turn stimulate the emission of even more photons. All of this emitted light has the same energy and thus the same wavelength. Light of a single wavelength or color is called monochromatic.
This chain reaction is effectively how lasers work. Atoms are “pumped” or a large number of their electrons are forced into energy levels usually 2-3 levels higher than the ground state. This pumping is done through either powerful flashes of light or through electric pulses.
Once the first photons are emitted, lasers additionally have two mirrors that act to reflect that stimulated light back and forth across the lasing medium (aka, the atom with all the excited electrons). By sending the emitted photons that have just the right amount of energy back through the pumped atom, those photons in turn stimulate the emission of even more photon pals. Then it’s a photon party!
Laser Guide Stars
 The focused nature of lasers makes them ideal tools for cutting when precise, sharp lines are needed. This requirement frequently comes into play when dealing with the human body, whether it’s sculpting our corneas for better vision, reshaping a chipped tooth, or removing a tattoo.
The focused nature of lasers makes them ideal tools for cutting when precise, sharp lines are needed. This requirement frequently comes into play when dealing with the human body, whether it’s sculpting our corneas for better vision, reshaping a chipped tooth, or removing a tattoo.
However, one of the coolest uses I’ve seen for lasers is in astronomy. When astronomers view a distant object like a star through our telescopes, the light we observe gets distorted as it passes through the Earth’s turbulent and messy atmosphere. Sometimes it can be difficult to decipher whether a distortion in our image is intrinsic to the star or if it’s just a viewing effect that should be subtracted out.
One way to get around this problem is the technique of adaptive optics: Astronomers observe their object of interest at the same time they observe a very bright object, usually a nearby star. Since they already know the image to expect from the comparison object, they look at the image they actually observe and then model what the atmosphere is doing. That atmospheric model is then used to adapt the optics of the telescope in real time to balance out the effects of the atmosphere when observing their target.
However, there is not always a nice, bright comparison object close by what you want to observe. When this happens, astronomers can just make their own star by shooting a laser up into the sky! These man- (or woman)-made stars are called laser guide stars and can be aimed anywhere they are needed.
This episode of Everyday Einstein was inspired by a fun science and technology quiz on Pew Research Center’s website that allows you to see how your results stack up in direct comparison to other quiz takers divided by gender and age group. The question about lasers was the second most likely question to be answered incorrectly!
In case you’re curious, the question most often answered incorrectly is which gas makes up most of the Earth’s atmosphere. Most folks think it’s oxygen but it’s in fact nitrogen.

Until next time, this is Dr. Sabrina Stierwalt with Everyday Einstein’s Quick and Dirty Tips for helping you make sense of science.
You can become a fan of Everyday Einstein on Facebook or follow me on Twitter, where I’m @QDTeinstein. If you have a question that you’d like to see on a future episode, send me an email at everydayeinstein@quickanddirtytips.com.
We’ve all been there. You’re listening to a podcast on the go when you hear a super interesting ad for a product or service, but simply don’t have the time to check it out. Well, now you’re in luck. See the full list of offers, discounts, and more from our Quick and Dirty advertisers over at quickanddirtytips.com/offers.
Tidak ada komentar:
Posting Komentar